Innovative Influenza mRNA Vaccine: A Step Towards Universal Protection
Written on
Chapter 1: Introduction to the Influenza mRNA Vaccine
Recent findings from a Phase I trial indicate that an experimental mRNA vaccine for influenza is both safe and effective. The vaccine prompted an increase in antibodies among all 180 adult participants.
The initial results were disclosed by Moderna, the U.S.-based biotechnology firm developing the vaccine. This new formulation utilizes the same mRNA technology that proved successful in their COVID-19 vaccine. According to Moderna's CEO, Stephane Bancel, “Even prior to the COVID-19 pandemic, around 3 million individuals lost their lives each year to viral respiratory infections, with countless others hospitalized due to complications.”
Bancel expressed optimism that this new influenza vaccine will outperform existing options and hopes it may eventually offer broad protection against various respiratory viruses.
Section 1.1: Phase I Study Overview
The Phase I trial primarily assessed the safety of the mRNA vaccine in humans. A range of doses was administered to the 180 participants, who varied in age, while side effects were closely monitored. Although the vaccine's ability to elicit an immune response was evaluated, its effectiveness in preventing actual infections remains untested.
Participants across all dosage levels exhibited an increase in antibodies specific to influenza viruses. The reported side effects were generally mild, with younger adults experiencing them more frequently than older ones. Common complaints included pain at the injection site, headaches, muscle and joint aches, and fatigue.
We will have to wait several months for complete results as subsequent phases of the study are conducted. A Phase II trial began last month to find the optimal dose and compare Moderna’s vaccine with traditional flu vaccines. This phase will involve 500 participants, with preliminary findings anticipated in early 2022. Future phases will assess the vaccine's effectiveness across different demographics.
Section 1.2: The Future of mRNA Vaccines
Current influenza vaccines primarily rely on inactivated viruses cultivated in chicken embryos, typically formulated as trivalent or quadrivalent vaccines. The strains included must be selected six to nine months before deployment, with effectiveness rates hovering around 40-60%.
Manufacturers like Moderna aim to leverage mRNA technology to significantly boost protection against influenza. A key advantage of this approach is the ability to incorporate multiple mRNA sequences targeting various strains within a single vaccine, potentially eliminating the need for seasonal vaccinations.
Moderna is also exploring other experimental vaccines, including a combination vaccine designed to protect against influenza, COVID-19, and respiratory syncytial virus (RSV). RSV poses a particular risk to infants, the elderly, and those with chronic health conditions.
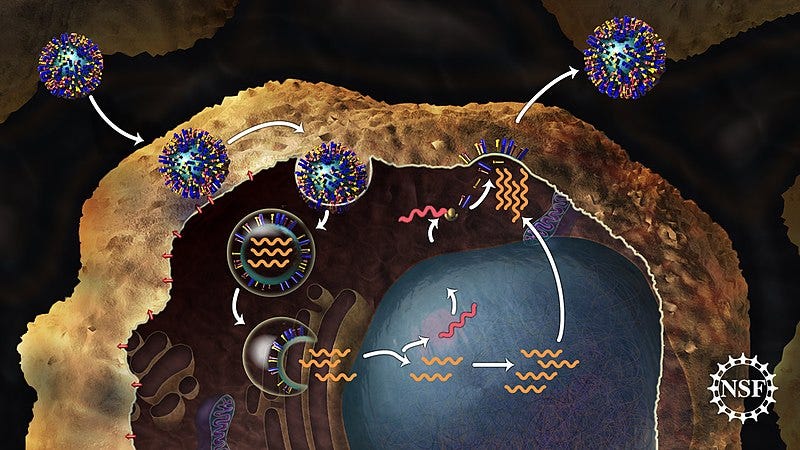
Chapter 2: Advancements in mRNA Technology
The rapid advancement of mRNA technology following the success of COVID-19 vaccines has sparked significant interest in developing an HIV vaccine. Recent reports from the U.S. National Institute of Allergy and Infectious Diseases (NIAID) reveal that an experimental mRNA vaccine for HIV has successfully completed trials in mice and primates.
“Despite nearly four decades of efforts by the global research community, an effective vaccine to prevent HIV remains elusive. This experimental mRNA vaccine incorporates several innovative features that may address the limitations of previous formulations, making it a promising candidate,” stated NIAID Director Anthony S. Fauci, PhD.