Exploring the Wonders of Lithium: A Fascinating Metal
Written on
Chapter 1: Introduction to Lithium
Lithium has been making headlines frequently, prompting me to explore this remarkable metal and its diverse applications.

I must confess, chemistry was never my favorite subject in school. I found it frustrating and imprecise, especially when compared to the hands-on experiments in physics that captivated me. While I was engrossed in understanding electricity and the mechanics of the universe, chemistry felt more like alchemy. However, even during my electrical experiments, the need for batteries reminded me of chemistry's unavoidable relevance.
Driven by curiosity, I set aside my aversion to chemistry to learn more about this intriguing metal, while trying to limit the complexities of its science.
Basics of Lithium
Lithium is a soft, silvery-white metal known for its lightweight properties, being the least dense solid element. It boasts a relatively high melting and boiling point for a metal and reacts vigorously with water.
In the periodic table, lithium is classified as element number 3, indicating it has three protons in its nucleus. Its atomic symbol, Li, derives from the Latin word "lithos," meaning stone. As an alkali metal, it resides in Group 1 alongside sodium, potassium, rubidium, cesium, and francium, sharing characteristics like low density and high reactivity with water.
Although my chemistry teacher's demonstration of sodium's reaction with water didn't spark my enthusiasm, I still find lithium's properties fascinating.
Scientific Discovery of Lithium
Lithium is typically found in nature bonded with other elements such as oxygen, silicon, and fluorine. In 1817, Johann Arfwedson successfully isolated lithium through electrolysis while examining mineral samples from Sweden. By 1855, Sir Humphry Davy had also isolated lithium using the same technique.
Where is Lithium Found?
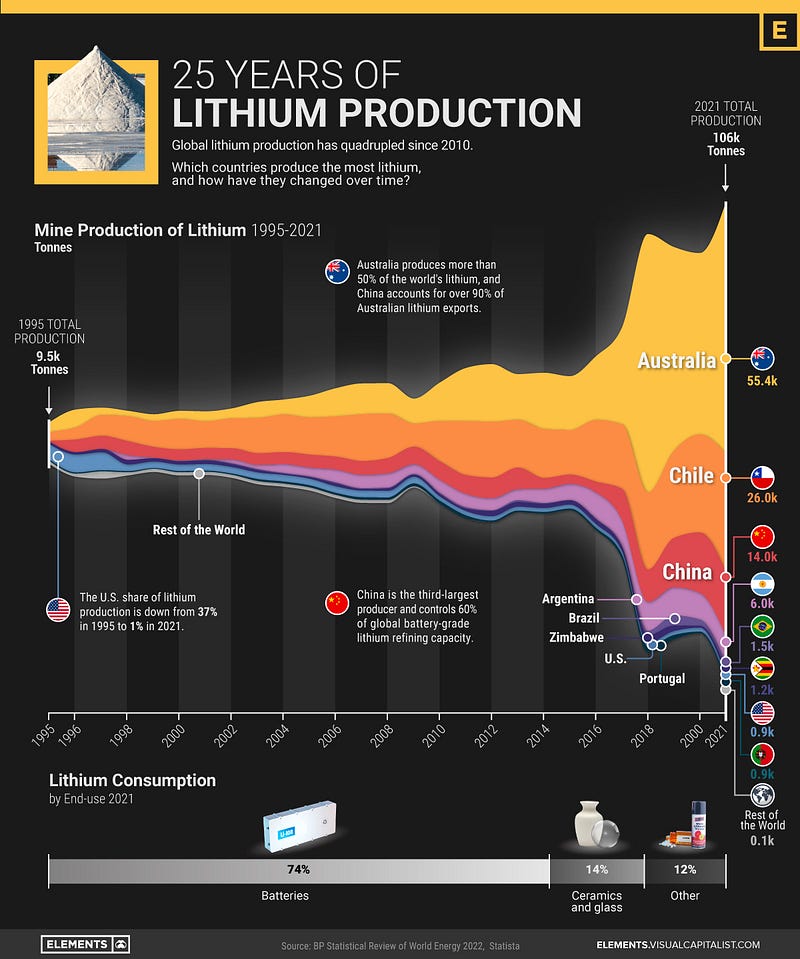
According to the US Geological Survey, the leading lithium-producing nations in 2019 were Australia, Chile, China, and Argentina. The region called the Lithium Triangle, formed by the intersection of Chile, Bolivia, and Argentina, is particularly rich in lithium resources. Notably, a deposit discovered in Wyoming in 2013 is estimated to contain about 228,000 tons, with additional deposits potentially amounting to 18 million tons, as reported by mining.com. Statista estimated that lithium production reached 100,000 tonnes in 2021, although some argue that its extraction methods can be environmentally harmful.
Lithium's Applications
A significant portion, around 56%, of lithium produced annually is dedicated to battery production, which merits special attention.
Lithium batteries are increasingly becoming a topic of discussion, especially in electric vehicles, which typically contain 8–10 kg of lithium batteries. On my boat, I still rely on deep-cycle lead-acid batteries, but lithium's presence is growing, particularly in the safer, lower-energy-density form of lithium iron phosphate (Li–FePO4).
Lead-acid batteries offer only about 15% of the recharge cycles that lithium batteries can provide, along with significantly less recoverable energy. Currently, I'm recharging my lead-acid batteries with a generator while also utilizing solar panels, and soon, a wind generator. Despite their effectiveness, lead-acid batteries last for about 500 cycles, whereas lithium iron phosphate batteries can last up to 3,000 cycles. Ultimately, lithium solutions yield a total cost of ownership per usable kWh that is approximately 2.8 times lower than lead-acid alternatives, according to Powertech Systems.
Fortunately, recent advancements have reduced the risk of fires caused by lithium batteries in airplanes.
At present, lithium-ion batteries average around $132 per kWh. Perhaps I should wait a little longer before switching to lithium.
Other Uses of Lithium
Beyond batteries, lithium finds application in various sectors, such as alloys, glass-ceramic products, explosives, and pharmaceuticals.
In the pharmaceutical realm, lithium is utilized to manage and prevent manic episodes in individuals with bipolar disorder. It's classified as an antimanic agent and has been shown to lower the risk of suicide. While it is an essential trace element for human health, it is typically found in drinking water at levels too low to provide therapeutic benefits.
Moreover, lithium enhances aluminum for aircraft parts and improves copper’s electrical conductivity. Its presence in glass ceramics makes cookware and ovenware highly heat-resistant. Surprisingly, lithium is also utilized in explosives, fireworks, and flares, usually in the form of lithium nitrate (LiNO3), which can be explosive in nature.
And Finally
Lithium even plays a role in the maintenance of my boat, specifically in the lithium-based grease found in the stern gland. This grease contains lithium stearate and lithium 12-hydroxy-stearate, providing both water resistance and thermal stability.
If I ever experience a bout of depression, I know where to turn—though I wouldn't recommend using grease for that purpose! While lithium may not possess magical properties, its versatility and medicinal uses certainly make it a captivating metal.
About Me
If you follow my journey, expect a diverse array of topics in your inbox, including humor, technology, space, geopolitics, and travel, along with daily musings from my life on a boat.
